In the work I do, I've come to see how profoundly global politics now intersect with the technical realities of mineral exploration. Amid growing geopolitical tensions, the way we think about the world's mineral reserves and where they're located, is being fundamentally reassessed. The trust in global economic stability that shaped the supply chains of past decades has eroded, and recent wars have profoundly shifted attitudes toward long-standing alliances. As a result, access to certain raw materials is no longer seen as a given.
In response to these changes, the European Union has introduced the Critical Raw Materials Act (CRMA), a strategic framework that classifies materials essential to the EU's economy and technological development-many of which are mineral-based. These are materials considered crucial for manufacturing everything from semiconductors to batteries and wind turbines. The CRMA not only identifies which materials are critical, but also emphasizes the importance of securing their supply chains through recycling, diversification, and, increasingly, domestic extraction within the EU. This has accelerated interest in both mineral exploration and the re-evaluation of previously overlooked deposits.
At the same time, I see a growing societal awareness of environmental limits, overconsumption, and the urgency of the green transition. These pressures don't make mineral exploration less important, they make it more demanding. We're being asked to find more, extract more, and do it better: with greater precision, transparency, and minimal environmental impact. That means it's no longer enough to know that a deposit exists, we must understand what exactly is in the ground, in what quantities, and how it's structured on a micro and macro scale.
That's where X-ray-based analytical techniques come into the picture. From my perspective, these methods offer a unique combination of non-destructive analysis, high spatial resolution, and sensitivity to both elemental composition and crystalline structure. They allow me to examine ore samples without altering them, to distinguish between closely related mineral phases, and to quantify material components with a level of detail that few other methods can match. Whether working with laboratory data or synchrotron data, the ability to extract both compositional and structural information from the same sample is invaluable, not only for exploration, but also for assessing resource potential and guiding environmentally responsible extraction strategies.
This text, designed for technical audiences, brings together the principles and applications of X-ray radiation sources and measurement setups from which the data is used in analytics. It serves both as a technical foundation and a reflection on why these methods matter in today's shifting geopolitical and environmental landscape. This overview deliberately concentrates on X-ray techniques; electron-microscopy methods such as SEM are beyond the scope of my routine workflow.
What are X-rays?
X-ray radiation is a form of high-energy electromagnetic radiation, meaning it consists of electromagnetic waves that carry a large amount of energy per photon. Electromagnetic radiation spans a spectrum that includes radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays. The energy of this radiation is directly related to its frequency and inversely related to its wavelength, so the shorter the wavelength, the higher the energy. X-rays have wavelengths ranging from approximately 10 picometers (that is, 1 × 10⁻¹² metres) to 10 nanometers, placing them at the high-frequency, high-energy end of the spectrum. These wavelengths are shorter than those of visible light but longer than gamma rays, giving X-rays a unique position in the electromagnetic spectrum [1]. This high energy enables X-rays to penetrate materials that are opaque to visible light and to interact with matter at the atomic scale. The radiation itself consists of photons, the quantized particles of the electromagnetic field, each carrying a specific amount of energy depending on its wavelength.
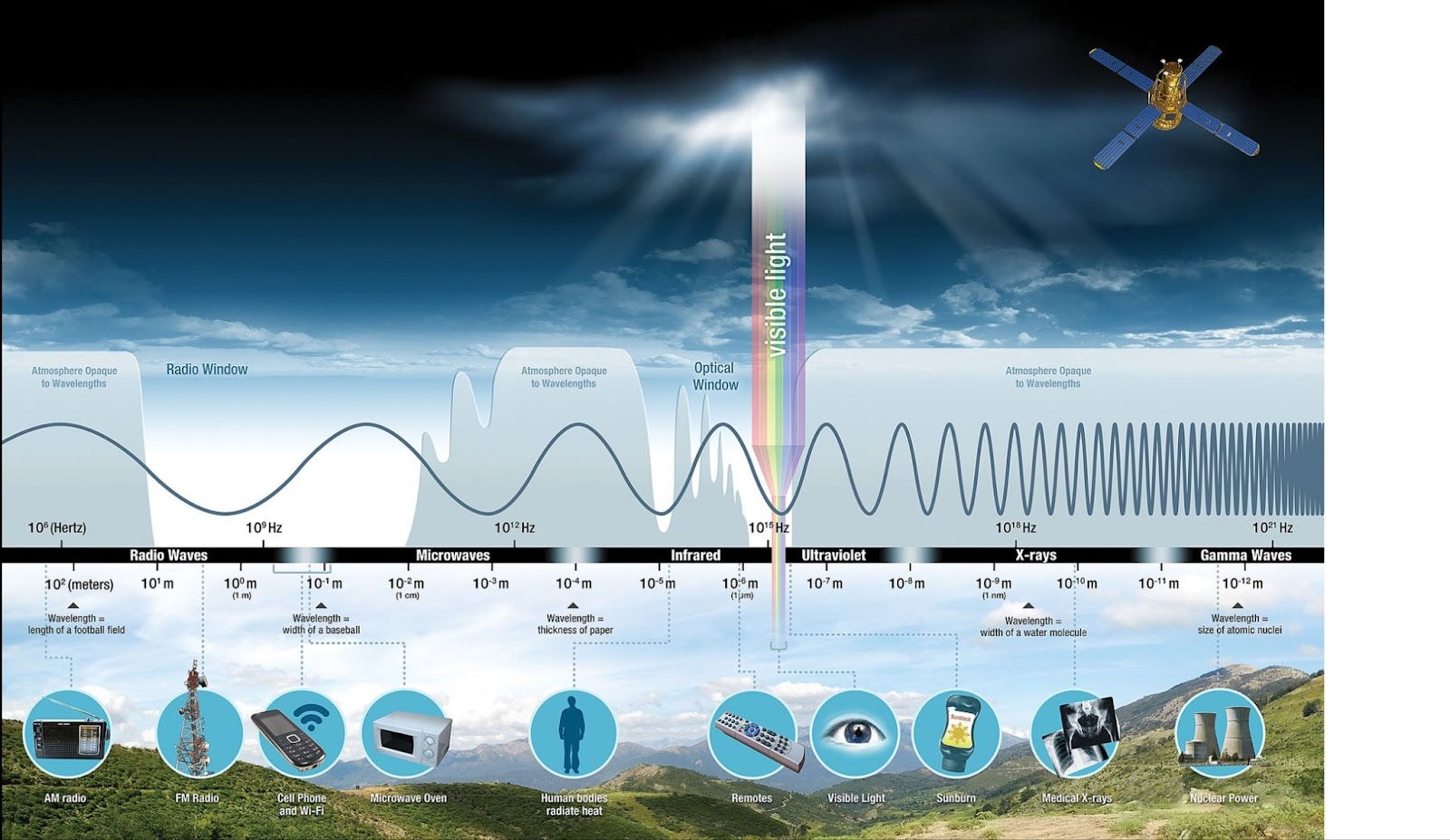
Electromagnetic spectrum. Source: NASA
X-rays are typically produced through two primary mechanisms. The first is bremsstrahlung, or braking radiation, which occurs when high-speed electrons are rapidly decelerated upon striking a dense target material inside an X-ray tube. The tube contains a heated cathode, which emits electrons through thermionic emission, and a metal anode, which serves as the positively charged target. Electrons are accelerated across a high-voltage potential and, when they collide with the anode, their rapid deceleration produces a continuous spectrum of X-ray radiation. The type of anode material defines the characteristic wavelength of the emitted X-rays. Copper, with a Kα wavelength of 1.5406 Å, is the most common anode material for mineralogical applications [2]. Cobalt, with a longer wavelength of 1.7902 Å, is often selected for samples containing iron, as it minimizes fluorescence effects that would otherwise interfere with the signal. In specialized cases, heavier elements such as molybdenum or silver are used, particularly for dense samples or advanced techniques [4][5]. A critical consideration is that the energy of the incident radiation should not exceed the absorption edge of any elements in the sample that might fluoresce. For example, copper radiation can excite iron atoms and increase background noise, whereas cobalt radiation avoids this problem and yields cleaner data [1][2][5]
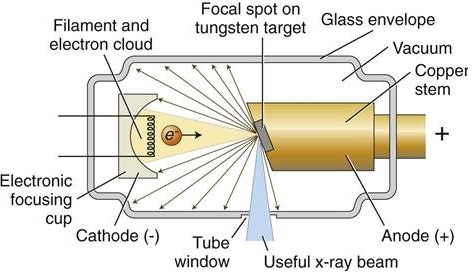
X-ray tube. Source: ResearchGate
The second mechanism is the generation of characteristic X-rays, which occurs when an incident electron from the cathode has enough energy to eject an inner-shell electron from an atom within the anode material. This creates an unstable electronic configuration. An electron from a higher energy shell then transitions inward to fill the vacancy, releasing the excess energy as a photon with a specific energy value. These emissions appear as sharp spectral lines, such as the Kα or Kβ lines and are unique to the atomic structure of the anode element [2].
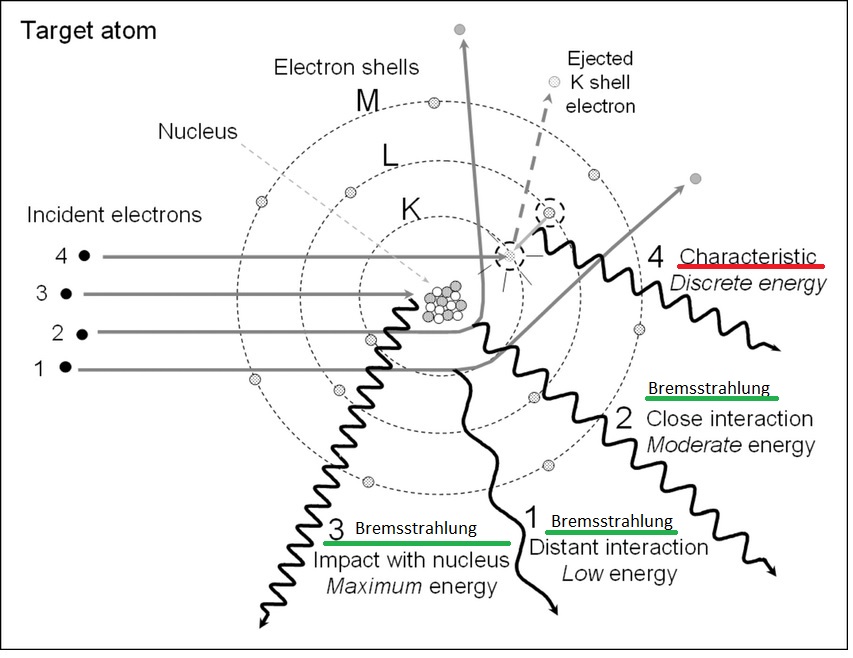
Bremsstrahlung (green) (1,2,3) and characteristic radiation (red) (4) mechanisms: the incident electrons interact with the target atoms resulting in X-ray production [14]. Source: ResearchGate
When X-ray photons encounter matter, several interactions are possible. One of the most important in crystallographic analysis is elastic scattering, in which the energy of the photon remains unchanged. This type of interaction forms the foundation of X-ray diffraction (XRD), which detects the scattering from an ordered atomic lattice and relies on the principle of Bragg's Law, which will be detailed below [3]. In contrast, inelastic scattering, such as Compton scattering, involves a change in photon energy and is not useful for structural determination. X-rays can also be absorbed by atoms, leading to the emission of secondary radiation known as fluorescence, which forms the basis of X-ray fluorescence (XRF).
X-ray diffraction
X-ray diffraction, also known as XRD or XRPD (X-ray powder diffraction), is based entirely on the elastic scattering of X-rays from crystalline materials. When a beam of X-rays strikes a regularly ordered lattice of atoms, constructive interference occurs at specific angles. This produces diffraction peaks that can be measured and interpreted to reveal the crystal structure and identify the mineral phases present. The phenomenon is governed by Bragg's Law, expressed as nλ = 2dsinθ, where n is the order of reflection, λ is the wavelength of the incident beam, d is the spacing between atomic planes, and θ is the angle of incidence. Diffraction occurs only when these conditions are met, meaning the X-ray beam must strike the lattice at a precise angle for the reflection to be detected. By scanning over a range of angles, one obtains a diffraction pattern that is effectively a fingerprint of the sample's crystal structure. These patterns are then compared to known entries in reference databases [2][3].
The geometry of an XRD measurement plays a crucial role in determining the accuracy and suitability of the collected data. One of the most commonly used geometries in laboratory instruments is the Bragg-Brentano, or θ-2θ reflection geometry. In this setup, the sample remains stationary while the X-ray source and detector move in a coordinated way, with the detector sweeping through twice the angle of the incident beam. This configuration is ideal for flat samples, such as powders pressed into pellets or gently spread onto sample holders [1][2].
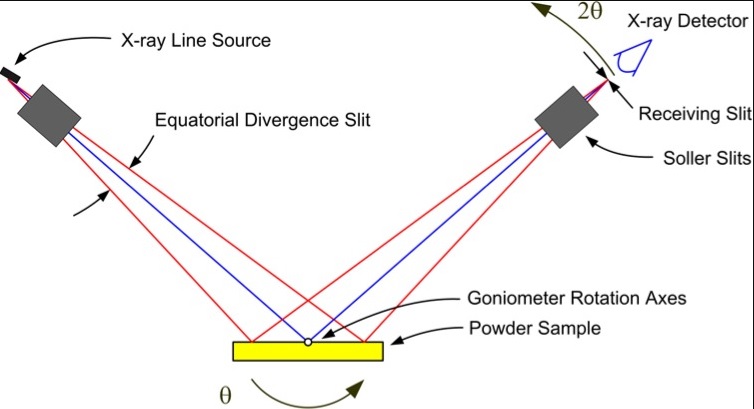
Bragg-Brentano Geometry. Source: SERC
Transmission geometry offers a different approach, where the X-ray beam passes through a thin layer of the sample. This method is particularly advantageous when minimizing surface effects or when only a small quantity of sample is available. The sample may be mounted as a thin film on a polymer support or sealed inside a glass capillary. Transmission setups are especially effective in synchrotron beamlines, which provide extremely collimated and intense X-rays [4].
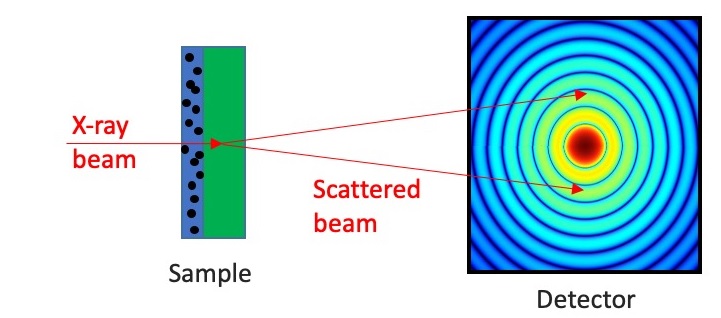
Transmission geometry. Source: Finden
Closely related to transmission geometry are capillary techniques, in which powdered samples are loaded into narrow glass tubes that rotate during measurement. This rotation ensures a more random orientation of the crystallites and helps reduce texture effects that might otherwise distort the diffraction pattern. These capillary methods are employed both in laboratory settings and at synchrotron facilities [1][4].
Another widely used configuration is parallel beam geometry, where the X-ray beam is conditioned using collimating optics-such as a Göbel mirror or a monochromator-to produce nearly parallel rays. This geometry is especially well-suited to samples with uneven or curved surfaces, such as geological specimens, metal coatings, or natural rocks. It minimizes geometric distortions and enhances angular precision [5]. The Göbel mirror itself is an important component in this setup. It is a multilayer optic, typically made from alternating layers of materials such as molybdenum and silicon, designed to reflect X-rays at specific angles. The result is a tightly collimated beam with extremely low divergence, often less than 0.01° [3].
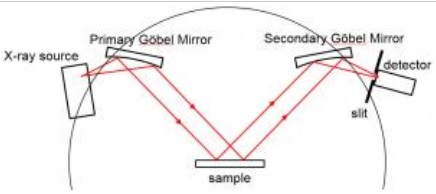
Parallel beam geometry. Source: SERC
Parallel beam geometry is particularly useful in challenging applications, such as grazing incidence XRD (GIXRD) and residual stress measurements, or when analyzing samples that cannot be flattened or pelletized. While it typically results in lower scattered intensity compared to focusing geometries, its precision and control over angular artifacts make it a preferred choice in many advanced applications [2][4]. The most effective configurations often combine a Göbel mirror with a monochromator on the X-ray tube and a set of slits or collimators on the detector side, forming a complete parallel beam setup that is compatible with both high-resolution laboratory systems and synchrotron sources.
The choice of sample type and crystallinity, whether a powdered material or a single crystal, determines the form of analysis. Powder diffraction (XRPD) can identify phases, estimate crystal structures, and determine lattice parameters. Single crystal XRD, on the other hand, provides a much more detailed analysis of atomic positions, symmetry, and unit cell parameters, and is commonly used in structural chemistry. However, single crystal methods are often impractical for most natural mineral samples due to their complexity or lack of suitable single grains [3][4]. In these cases, diffraction patterns from single crystals appear as discrete spots in a 3D array, and Fourier analysis can be applied to reconstruct atomic positions, making this method indispensable in structural studies.
For a detailed explanation of how anode material determines characteristic X-ray lines—and why Cu, Co, Mo, or Ag are chosen—see the X-ray generation section above. A critical consideration is that the energy of the incident radiation should not exceed the absorption edge of any elements in the sample that might fluoresce. For example, copper radiation can excite iron atoms and increase background noise, whereas cobalt radiation avoids this problem and yields cleaner data [1][2][5].
X-Ray Fluorescence
While XRD is primarily concerned with identifying the crystal structures and phases of minerals, X-ray fluorescence (XRF) provides complementary information by determining the elemental composition of solid materials. XRF is a non-destructive technique based on the excitation of atoms by incident X-rays. When an inner-shell electron is ejected from an atom, the vacancy is filled by an outer-shell electron, and the energy difference is emitted as a photon. These emitted photons have characteristic energies specific to each element, enabling precise elemental identification [1].
XRF is often described as rapid, non-destructive, and capable of operating with minimal sample preparation. In contexts such as field screening or preliminary assessments, this is true—samples can be analyzed in their raw form, including unprepared powders, rock chips, or pressed pellets. However, when accurate and reproducible quantitative results are required, particularly in laboratory settings, sample preparation becomes significantly more involved. Achieving high analytical precision often necessitates thorough grinding to produce a fine, homogeneous powder, followed by careful weighing and, in many cases, fusion of the sample into glass beads using platinum crucibles. This process helps eliminate particle size effects, reduce mineralogical matrix influences, and improve sample stability and uniformity.
Poorly prepared or heterogeneous samples introduce significant uncertainty into the analysis. Uneven surfaces may scatter the incident X-ray beam irregularly, while compositional inhomogeneities lead to matrix effects, interactions between coexisting elements that alter fluorescence intensities and distort the apparent concentrations of target analytes [2]. These effects are particularly problematic in complex geological matrices and must be corrected or minimized through proper sample handling.
The choice of instrumentation also determines the degree of necessary preparation and the type of data obtained. Energy-dispersive X-ray fluorescence (EDXRF) detects emitted photons based on their energy, using a solid-state detector. It is fast, compact, and well-suited to portable or benchtop systems. EDXRF is widely used for qualitative and semi-quantitative analysis, especially in fieldwork and rapid industrial screening, where flexibility and speed are prioritized over absolute accuracy. However, its spectral resolution is lower than that of wavelength-dispersive systems, which can limit its ability to resolve overlapping peaks in complex samples.
In contrast, wavelength-dispersive X-ray fluorescence (WDXRF) uses a crystal diffraction system to separate emitted X-rays by wavelength before they reach the detector. This allows for much higher spectral resolution, enabling better separation of closely spaced peaks and yielding improved accuracy, sensitivity, and lower detection limits, particularly for light elements. WDXRF is the preferred method in high-end laboratory settings where precise, quantitative, and reproducible data are required. However, this accuracy comes at the cost of longer measurement times, higher equipment complexity, and the need for carefully prepared, homogeneous samples [3].
It is also important to note that XRF determines elemental composition but not chemical bonding or mineral phase. For example, it cannot distinguish between iron present in magnetite, hematite, or siderite. For this reason, XRF is frequently paired with X-ray diffraction (XRD), which provides complementary information about the crystallographic structure and enables phase-specific identification [1][3].
Synchrotron radiation
Synchrotron radiation represents a further enhancement in X-ray-based mineral analysis. Produced by accelerating electrons to nearly the speed of light and then bending their paths with magnetic fields, synchrotron X-rays are extremely intense, highly collimated, and tunable across a wide energy range. Their brightness and coherence far exceed those of laboratory sources, making them valuable in applications ranging from materials science and chemistry to biology and geoscience [3].
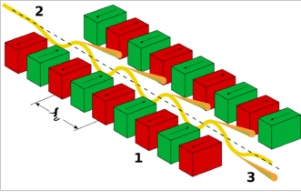
Working of the undulator: 1 magnets 2 electron beam entering from the upper left 3 synchrotron radiation exiting to the lower right. Source: Wikipedia
One of the key advantages of synchrotron radiation is its ability to detect weak diffraction peaks and subtle signals in mildly amorphous or highly complex samples. This makes synchrotron-based XRPD particularly effective for identifying trace phases and rare minerals [4]. Another powerful technique is microbeam XRF (μXRF), which focuses the beam to micrometer scales, allowing spatially resolved elemental mapping. This is especially useful in visualizing mineral zoning, identifying inclusion boundaries, and studying sulfide distributions in ore samples [3].
Collimated beam. Source: Wikipedia
Despite its advantages, synchrotron analysis is not without limitations. Access is restricted to large research facilities, and data interpretation often requires specialized software and expertise. Nevertheless, the high-quality data produced in these settings has additional value. It can be used to train machine learning algorithms for automated mineral analysis, accelerating the pace of discovery and improving routine analytical workflows [4].
Conclusion
In my experience, the intersection of mineral exploration, geopolitics, and sustainability is no longer theoretical-it defines the very conditions under which we now operate. The urgency to secure critical raw materials, as emphasized by the CRMA, has elevated the importance of technical precision in assessing what lies beneath the surface. At the same time, the environmental and societal pressures surrounding resource extraction are demanding more than just quantity, they require quality of knowledge, accountability, and scientific transparency.
This is why I rely so heavily on X-ray-based analytical methods. They allow me to approach mineral samples not just as geological curiosities, but as complex systems whose value, structure, and potential must be fully understood before decisions are made. These techniques-XRD, XRF, and their synchrotron-enhanced counterparts, have proven to be among the most versatile and insightful tools available. They help me identify minerals, quantify elements, map structures, and even anticipate processing behavior, all while preserving the integrity of the material.
As the demand for critical raw materials grows, and as exploration moves into increasingly complex geological, political, and environmental terrain, the ability to produce reliable, high-resolution data will only become more central. X-ray methods don't just answer technical questions, they contribute to broader decisions about sourcing, sustainability, and sovereignty.
In that sense, this text has not only laid out the principles behind these techniques but also reflects the reasons I choose to use them. For me, they are not just methods-they are part of a larger strategy to make mineral exploration both more intelligent and more responsible in a time when that balance is more essential than ever.
References
[1] Cullity, B.D. & Stock, S.R. Elements of X-Ray Diffraction, 3rd ed., Prentice Hall, 2001.
[2] Jenkins, R. & Snyder, R.L. Introduction to X-ray Powder Diffractometry, Wiley-Interscience, 1996.
[3] Als-Nielsen, J. & McMorrow, D. Elements of Modern X-ray Physics, Wiley, 2011.
[4] Klug, H.P. & Alexander, L.E. X-ray Diffraction Procedures, Wiley, 1974.
[5] Beckhoff, B. et al. Handbook of Practical X-ray Fluorescence Analysis, Springer, 2006.
[6] Jenkins, R. X-ray Fluorescence Spectrometry, 2nd ed., Wiley-Interscience, 1999.
[7] Van Grieken, R. & Markowicz, A. Handbook of X-ray Spectrometry, Marcel Dekker, 2002.
[8] Ortega, R. et al. (2009). Micro-XRF and synchrotron-based elemental imaging of biological samples. TrAC Trends in Analytical Chemistry, 28(11), 1300–1312.
[9] Scarlett, N.V.Y. & Madsen, I.C. (2006). Quantitative phase analysis using the Rietveld method – a practical examination of issues. Powder Diffraction, 21(4), 278–284.

